
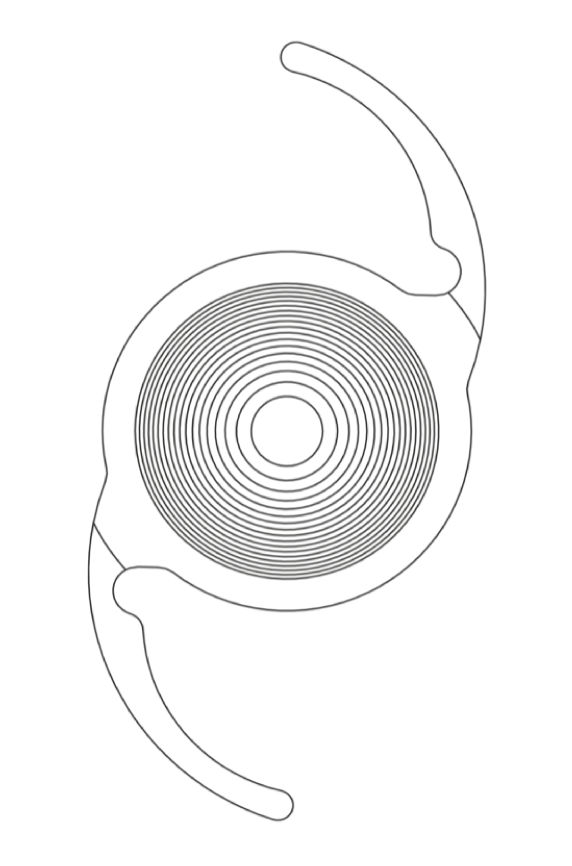
TECNISTM Multifocal 1-Piece IOL ZLB00
Diffractive multifocal lens1
*Value theoretically derived for a typical 20.00 D lens. Johnson & Johnson Vision recommends that surgeons personalize their A-constant based on their surgical techniques and equipment, experience with the lens model and postoperative results.
†Derived from clinical evaluation results of the TECNISTM 1-Piece Platform.
For healthcare professionals only. Please reference the Instructions for Use for a complete list of Indications and Important Safety Information and contact our specialists in case of any question.
© Johnson & Johnson Surgical Vision, Inc. 2022. PP2022MLT4910